Company Overview: A drug advancement company, Opthea Limited (ASX: OPT) is involved in expanding innovative, biologics-centered therapies for the treatment of eye infection. The company’s intellectual property is held within its wholly owned subsidiary Vegenics Pty Ltd. Its product development programs are primarily aimed at improving OPT-302 for wet age-related macular degeneration (wet AMD) and diabetic macular edema (DME). Further, the company remains committed to develop the vision of its patients who are suffering from retinal eye diseases.
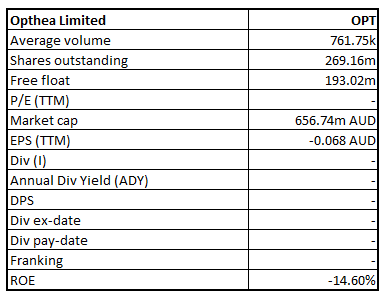
OPT Details
(48).png)
Strong Liquidity Position & Higher Investment are Key Growth Catalysts: Opthea Limited (ASX: OPT) is engaged in developing and commercialising therapies, primarily for eye disease. The company is engaged in controlling global rights to a substantial intellectual property portfolio of VEGF-C, VEGF-D and VEGFR-3.
Recently, the company provided encouraging top-line results of its Phase 2a trial assessing safety and effectiveness of OPT-302 controlled with Eylea® (aflibercept) in treating refractory patients with constant diabetic macula edema (DME). Notably, approximately 52.8% of refractory DME patients earned more than or equal to 5 letters of gain in Best Corrected Visual Acuity at week 12, subsequent to the OPT-302 combination therapy. Further, the test results revealed that the company met a co-primary endpoint of safety with 2.0 mg OPT-302 in combination with 2.0 mg Eylea. The results of the Phase 2a trial of OPT-302 signifies a step forward for the treatment of patients with retinal eye diseases, such as wet AMD and DME. Hence, the positive outcomes of the study reflected a major milestone for Opthea and place the company as a key global player in ophthalmology.
In today’s world, several patients remain untreated and have very limited options for anti-VEGF-A therapies. The need for more effective and durable therapies for DME keeps increasing with time. The company’s OPT-302 holds potential to improve vision and meet the rising unmet needs of patients, suffering from such illnesses. Markedly, the company’s efforts to advance the clinical development of OPT-302 by progressing patient recruitment into the company’s Phase 2a clinical trial, demonstrate its strong financial position. Going forward, OPT is currently planning to rapidly advance OPT-302 into pivotal, registrational Phase 3 development, including regulatory engagement in the US and Europe, on the back of strong clinical data. OPT enjoys a decent financial position to undertake this planning and complete the DME trial.
Coming to the past three-years performance over the period covering FY16 to FY19, OPT witnessed a top-line CAGR of ~6.1%. This reflects that OPT can make deployments towards its business activities which might help it in tapping long-term growth prospects.
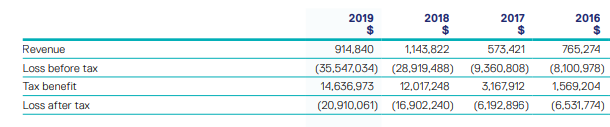
Historical Trends (Source: Company Reports)
1HFY20 Financial Highlights for the year ending 31 December 2019: OPT declared its interim results wherein, the company reported total revenues of $273,115, as compared to $480,338 reported in the year-ago period. During the period, the company reported a loss of $7,620,018, as compared to a loss of $11,281,819 reported in the year-ago period. In 1HFY20, majority of the expenditure of the group was on Research & Development (R&D) at $8,340,640 as compared to $17,352,777 in 1HFY19. Costs associated with R&D were related to the Phase 2a, Phase 2b and Phase 1b/2a clinical trials of OPT-302 for wet AMD and DME, and sourcing of standard of care anti-VEGF-A agents, used in the clinical studies. Other important expenses were related to patent and intellectual property costs, and administrative expenses that amounted to $203,982 and $2,416,266 as compared to costs of $149,768 and $2,073,462, respectively, in 1HFY19. During the period, the company received a R&D tax incentive claim of $14,636,973.

1HFY20 Key Financial Highlights (Source: Company Reports)
Improving Liquidity Position: The company reported $79.5 million of total current assets as at 31 December 2019. Cash and cash equivalents at the end of the period were reported at $75.1 million as compared to $21.5 million at the end of 30th June 2019. Receivables for the period were reported at $315,937, as compared to $295,786 at the end of 30 June 2019. Net tangible asset backing per share as on December 31, 2019 was reported at $0.27 as compared to $0.12 as at 30 June 2019. Net cash inflow from operating activities came in at $3,910,355 while net cash inflow from investing activities was $478,819.
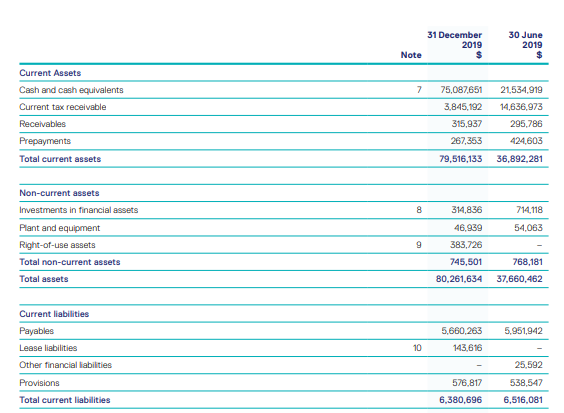
Balance Sheet Highlight (Source: Company Reports)
Capital Raising Remains a Key Catalyst: In December 2019, the company undertook a Capital Raising program. Notably, the company raised $50 million equity capital in a placement backed by Australian and UK based institutional investors. In September 2019, the company received a $14.6 million R&D tax credit from the Australian Taxation Office. The funds garnered under the offer make the company fully funded to carry out the completion of the Phase 2a clinical trial. Also, the funds would be deployed to progress Phase 3 preparatory activities, which incorporates the manufacturing of OPT-302 and initiation activities for a Phase 3 program in wAMD. Further, the available funds will also be used towards working capital, costs of the offer, further preclinical studies, etc. Henceforth, the fund raising will further strengthen liquidity position and makes it more effective to undertake business transactions which can prove to be beneficial over the long term.
Recent News: In a recent update, the company stated that it was added to the S&P/ASX 300 Index, effective upon market open on 22 June 2020. The company’s addition to the ASX 300 signifies its robust performance through its lead product candidate, OPT-302, and the completion of Phase 2 clinical trials.
Top 10 Shareholders: The top 10 shareholders have been highlighted in the table, which together form around 45.56% of the total shareholding. Regal Funds Management Pty. Ltd. is the entity holding maximum shares in the company at 10.67%. Baker Bros. Advisors LP is the second-largest shareholder, with a holding of 9.05%.
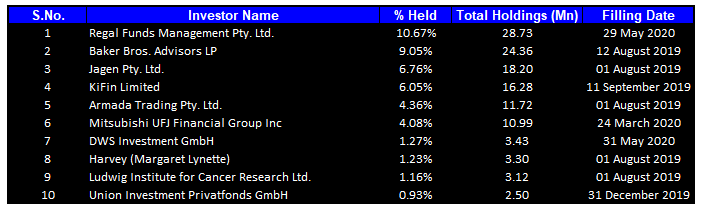
Top Ten Shareholders (Source: Refinitiv, Thomson Reuters)
Key Metrics: In 1HFY20, the company had a current ratio of 12.46x, higher than the industry median of 4.58x, representing a sound liquidity position. Debt to Equity ratio for 1HFY20 stood at 0.01x, lower than the industry median of 0.24x. The company is optimistic about business growth, looking at the potential contribution of clinical trials and lower debt levels.
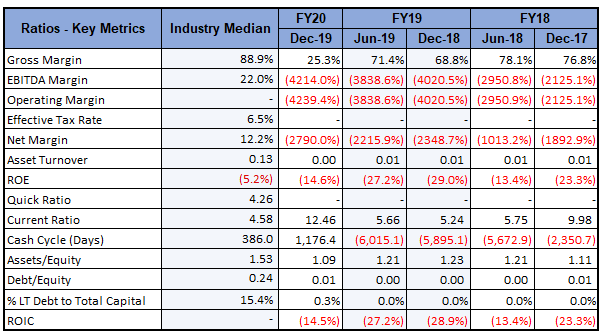
Key Metrics (Source: Refinitiv, Thomson Reuters)
Key Risks: On the flip side, pricing pressure in the competitive Australian healthcare market remains a headwind. Further, the company is also exposed to financial risks, liquidity risks and foreign currency fluctuation risks. Also, the increased costs related to developing a drug using a costly technology and pipeline setbacks are few major headwinds. Most drugs are years away from a potential approval that makes the returns more ambiguous. Other headwinds faced by the company include government scrutiny of high drug prices and stiff competition in the market.
Outlook: The company’s capital raising initiatives, and its focus towards delivering on the pipeline of catalysts aids the company’s future growth prospects. Moreover, the company is spending millions to improve its product pipeline with pathbreaking technology inducing a huge R&D expenditure. Positive data from the trials, encouraging pipeline progress and favorable regulatory updates are likely to aid the company in the near future. Further, mergers & acquisitions have been an essential trend in this vibrant sector as prominent pharma/biotech companies look to expand their revenue bases. In addition, coronavirus pandemic is unlikely to have a material long term impact on the business. Subsequent to the completion of its capital raise, OPT has reported a strong cash position that is likely to fully fund the company through to the completion of its phase 3 trials.
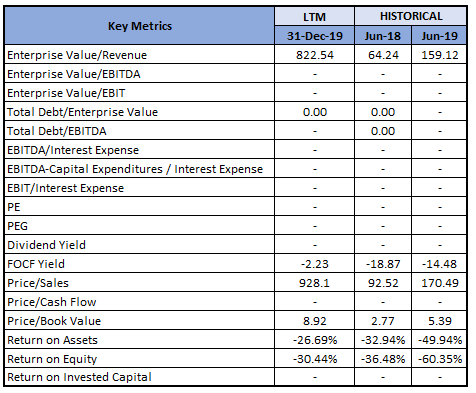
Key Valuation Metrics (Source: Refinitiv, Thomson Reuters)
Valuation Methodology: EV/Sales Multiple Based Relative Valuation (Illustrative)
.png)
EV/Sales Multiple Based Relative Valuation (Source: Refinitiv, Thomson Reuters)
Note: All forecasted figures and peers have been taken from Thomson Reuters, NTM-Next Twelve Months
Stock Recommendation: As per ASX, the share price has fallen ~17.57 per cent in the past six months as on July 07, 2020 while it has risen around 20.79 per cent in the last three months. Currently, it is trading near the average of its 52-week trading range of $0.735 - $4.15. The company’s positive outcomes of the Phase 2a study, along with a successful capital raising program remain a key growth catalyst. We have valued the stock using a illustrative relative valuation method, i.e., EV/Sales multiple, and arrived at a target price of low double-digit upside (in % terms). Hence, we recommend a “Buy” rating on the stock at the current market price of $2.44 as on 8 July 2020.
.png)
OPT Daily Technical Chart (Source: Refinitiv, Thomson Reuters)
Disclaimer
The advice given by Kalkine Pty Ltd and provided on this website is general information only and it does not take into account your investment objectives, financial situation or needs. You should therefore consider whether the advice is appropriate to your investment objectives, financial situation and needs before acting upon it. You should seek advice from a financial adviser, stockbroker or other professional (including taxation and legal advice) as necessary before acting on any advice. Not all investments are appropriate for all people. Kalkine.com.au and associated pages are published by Kalkine Pty Ltd ABN 34 154 808 312 (Australian Financial Services License Number 425376). The information on this website has been prepared from a wide variety of sources, which Kalkine Pty Ltd, to the best of its knowledge and belief, considers accurate. You should make your own enquiries about any investments and we strongly suggest you seek advice before acting upon any recommendation. Kalkine Pty Ltd has made every effort to ensure the reliability of information contained in its newsletters and websites. All information represents our views at the date of publication and may change without notice. To the extent permitted by law, Kalkine Pty Ltd excludes all liability for any loss or damage arising from the use of this website and any information published (including any indirect or consequential loss, any data loss or data corruption). If the law prohibits this exclusion, Kalkine Pty Ltd hereby limits its liability, to the extent permitted by law to the resupply of services. There may be a product disclosure statement or other offer document for the securities and financial products we write about in Kalkine Reports. You should obtain a copy of the product disclosure statement or offer document before making any decision about whether to acquire the security or product. The link to our Terms & Conditions has been provided please go through them and also have a read of the Financial Services Guide. On the date of publishing this report (mentioned on the website), employees and/or associates of Kalkine Pty Ltd do not hold positions in any of the stocks covered on the website. These stocks can change any time and readers of the reports should not consider these stocks as personalised advice.
AU


(48).png)






.png)
.png)
 Please wait processing your request...
Please wait processing your request...